News Story
New Device Models Will Improve Design of Biomedical Devices
Researchers who study artificial capsules in microcapillary and microfluidic devices now have easier ways to characterize their behavior thanks to a new pair of methodologies developed by University of Maryland associate professor Panos Dimitrakopoulos (Department of Chemical and Biomolecular Engineering). The recently published computational work describes and validates the designs of two experimental devices that can be fabricated in the laboratory and used to measure the shear and area-dilatation moduli of capsule membranes.
The study of these tiny, fluid-filled membranes and their behavior in viscous flows has steadily increased due to their versatility in engineering and biomedical applications. They are often used in pharmacy for targeted drug delivery, the food and textile industries, agriculture and cosmetics, and for the fabrication of microparticles with desirable properties. In addition, their behavior is similar to that of red blood cells flowing through blood capillaries.
“The ability to determine the elastic properties of the membranes of artificial capsules is essential to improving the design of the devices in which they are utilized,” explains Dimitrakopoulos. “We need to know how capsules will distort as fluid flow pushes them though tight spaces in microchannels, especially where there is a change in the width of their path.”
For example, he says, if someone is designing a drug delivery system, he or she needs to know under what conditions the capsule’s membrane will rupture and release its cargo due to excessive deformation as it travels through small blood capillaries.
The elasticity of a capsule membrane is characterized by its shear modulus (the resistance to a change in its shape but not its surface area) and its area-dilatation modulus (the resistance to an increase in its surface area without changing its shape). Acquiring this information from a tiny capsule inside an equally tiny microdevice isn’t easy. Shear and area-dilatation occur simultaneously as a capsule deforms, and calculating the former typically requires knowing the latter. This results in researchers having to use multiple devices and perform a significant amount of testing in order to separate shear from area-dilatation effects and calculate the value of the individual moduli.
Dimitrakopoulos’ group has designed two new approaches that streamline the capsule characterization process: one using microfluidic (channel-like) devices, and one using microcapillary (pipe-like) devices.
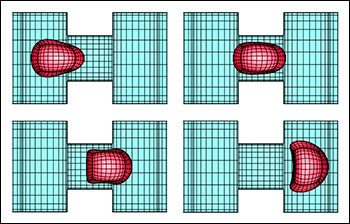
Dimitrakopoulos’ first methodology, published in the Journal of Fluid Mechanics, explains how to derive both the shear and area-dilatation moduli of a capsule in a single experiment conducted in a microfluidic device. The capsule is placed at the intersection of two channels in what is known as a “four-roll mill” device. As liquid flows through, it diverts around the capsule, splitting and turning left and right into perpendicular channels. These multidirectional flows both deform the capsule and create a “stagnation point” at the center of the intersection, holding it in place. The flow strength is increased to create greater distortions of the capsule’s width and length that can be recorded visually with a camera. By comparing these lengths with computational results obtained by Dimitrakopoulos’ group, the value of both elastic moduli can be derived.

Left: A diagram of the planar extensional fluid flow in a microfluidic device that holds and deforms a capsule at its center. Right: A model of the steady-state lamellar shape of a capsule with low membrane hardness while in the microfluidic device.
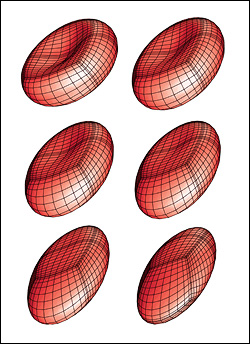
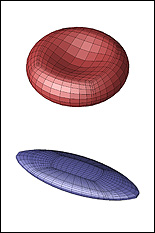
The second paper, published in Soft Matter, describes a new methodology for determining a membrane’s shear modulus, independent of its area-dilatation modulus, by flowing a capsule through a microcapillary device that suddenly narrows to a diameter comparable in size. The capsule’s length is measured from photos that capture it at its maximum deformation as it is forced to squeeze through this bottleneck. In its computational investigation, Dimitrakopoulos’ group showed that the capsule's maximum elongation is characterized by local shape-changing under a constant surface area, and therefore depends only on the membrane’s shear modulus. Comparing experimental measurements of the capsule's maximum elongation with computational results derived for the same microdevice, the membrane’s shear modulus is easily determined, without the need to know its area-dilatation modulus. According to Dimitrakopoulos, the proposed experimental device can be easily fabricated from glass, and with a continuous flow, it is possible to characterize a large number of artificial capsules.

A model showing the distortion of a capsule as it squeezes through a microcapillary device. Measurements used to determine its shear modulus are taken at its greatest distortion/elongation, which occurs as it passes through the bottleneck.
The new techniques are the latest developments in Dimitrakopoulos’ ongoing computational studies, which include the behavior of artificial capsules and red blood cells in microfluidic devices, the microcirculation of the human body and in disease states.
For More Information:
P. Dimitrakopoulos, “Effects of membrane stiffness and scaling analysis for capsules in planar extensional flows,” J. Fluid Mech., 745, 487–508 (2014). (Online)
P. Dimitrakopoulos and S. Kuriakose, “Determining a membrane’s shear modulus, independent of its area-dilatation modulus, via capsule flow in a converging microcapillary,” Soft Matter, 11, 2782-2793 (2015). (Online)
Published March 26, 2015