News Story
New Technique Turns Alginate Solution Into Micron-Scale Gel Patterns Using Light
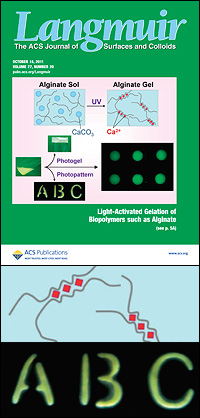
Top: "Light-Activated Ionic Gelation of Common Biopolymers" on the cover of Langmuir. Middle: An illustration showing how calcium ions lodge themselves between two molecular chains of alginate, causing the material to transform from solution to gel. Bottom: An example of the precise, sub-millimeter-sized gel structures Javvaji and his colleagues were able to create by exposing their modified alginate solution to light through a mask.
The paper was first-authored by Department of Chemical and Biomolecular Engineering (ChBE) graduate student Vishal Javvaji, and co-authored by his co-advisors, Professor Srinivasa Raghavan (ChBE) and Professor Gregory Payne (Fischell Department of Bioengineering and Institute for Bioscience & Biotechnology Research), and Aditya G. Baradwaj (B.S. '11, chemical engineering; currently a Ph.D. student at Purdue University).
Javvaji studies "smart materials"—fluids, gels and surfaces whose qualities can be changed when exposed to specific stimuli, such as heat and light. His recent work has focused on alginate, a common, natural polymer derived from seaweed that is used in everything from food to special effects makeup to supportive environments for cells used in tissue engineering.
Despite being a favorite of those engaged in biomaterials research, alginate is not a light-responsive material and would typically not be the main ingredient in the smart materials Javvaji and his colleagues create. However, by combining their knowledge of two pre-existing chemistry techniques, the team was able to not only get the alginate to gel when exposed to light, but also do it very precisely, in specific shapes exactly where they wanted them, leaving the remainder in liquid form.
Typically, an alginate solution is converted to a gel by the addition of calcium salt, which solidifies it into one mass. "If you want to make small structures out of a gel," says Raghavan, "then doing it that way is not really feasible. You can't mix chemicals in a beaker and take out a patterned structure, but people can and do create very precise gels using light. Our challenge was, how can we do it with this polymer that isn't naturally light-responsive?"
To solve the problem, Javvaji started with calcium carbonate (the chalky substance used in antacids), which unlike calcium salts is not water-soluble and, added to an alginate solution, will not cause it to gel. If particles suspended in the solution are exposed to acid, however, the resulting chemical reaction creates water-soluble free calcium ions, and gelling will occur.
Javvaji could not simply pour acid into the mixture because he would end up with the usual result: one big gel. So in his next step, he turned to a class of compounds called photoacid generators (PAGs). When exposed to ultraviolet (UV) light, these compounds undergo a chemical reaction that produces acid. A PAG molecule was added to the alginate/calcium carbonate solution.
Alginate is comprised of chains of molecules. When packed closely together, small empty zones are created where two chains are side by side—Javvaji describes it as looking a little like an empty egg carton. These voids, dubbed "egg box junctions," are the perfect size for calcium ions to nestle into. When UV light is shined on the solution, the PAG releases acid, the acid turns the calcium carbonate soluble, and free calcium ions lodge in the little spaces between the alginate chains, forming the weak bonds that turn the solution into a gel. If Javvaji exposes the solution to light through a mask, this will only occur where the light hits, allowing him to create millimeter- and sub-millimeter-sized patterns and shapes.
The gel can be returned to liquid form by adding calcium chelators, molecules that bind to the calcium ions, pulling them from the alginate chains and breaking the physical bonds.
"The ideas of combining calcium carbonate with acid to generate free calcium and using PAGs to generate acid with light have been out there in the literature," says Raghavan, "but Vishal was the first put the two concepts together."
"We're excited about the project because it allows simple and widely-used photolithographic methods to be employed to create structure with biological materials," Payne adds.
The team considers the work to date a fundamental proof-of-concept, and hopes it will generate interest among those who routinely use alginate in their research or products. Javvaji notes the concept could have broader applications. "This method of gelation is not specific to alginate," he explains. "We proved in the paper that it can be extended to pectin, another biopolymer [derived from fruit and used in food]. Anything that responds to calcium or any other salt can be gelled with light using this technique."
For More Information:
Visit Professor Raghavan's Complex Fluids and Nanomaterials Group web site »
Visit Professor Payne's homepage »
Published November 17, 2011