News Story
'Super Electrolyte' Capable of Operating in Extreme Temperatures, From the Arctic Tundra to the African Savannah
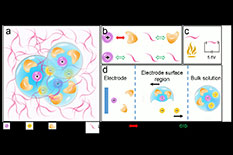
a) The electrolyte uses a non-polar solvent (D2 or M3, denoted by the purple curves) to tame the fluorinated carbonate electrolytes. The transparent blue spheres indicate the Li-ion solvation structure. The purple, positive-charged spheres indicate the Li-ions. The brown, negative-charged spheres are anions. The solvents with brown crescent shapes around the Li-ions are the fluorinated carbonate solvents. b) The affinities between the solvents and ions. The Li-ions and fluorinated carbonate solvents have a strong interaction (indicated by the solid red arrow), while the other three species have weak interactions between each other (dashed arrows). C) The non-flammable and high electrochemical stability requirements for the non-polar solvent in the super-electrolyte. The non-polar solvents are non-flammable and can withstand an extremely high voltage of 5.6 V. d) The expected electrochemical process at the electrode and electrolyte interface of the tamed electrolyte. In the bulk electrolyte, the Li-ions will be solvated by fluorinated carbonate molecules and anions. At the surface region, the solvated Li-ions will separate from the anions by means of the electric field. As the Li-ions arrive at the surface of the electrode, the fluorinated carbonate molecules will finally be dissolved.
There is an urgent call for high-energy lithium-ion batteries (LIB) that are safe, cheap, efficient and capable of operating in a wide temperature range. The demand stems not only from the instability and uncertainty of some mobile devices, but also from the forecasted surge of hybrid and electronic vehicles as well. Although extensive efforts are constantly made, one of the main problems with reported wide-temperature battery chemistry strategies is that they reduce energy and power density, and often sacrifice safety. Most LIBs currently on the market operate within a narrow temperature window — resulting from the strong bond between solvents and Li-ions — a characteristic required for high ionic conductivity, until recently.
A research team led by Chunsheng Wang, a professor in the University of Maryland (UMD) Department of Chemical and Biomolecular Engineering (ChBE), has developed a method of breaking the bond between solvents and Li-ions by dissolving the fluorinated carbonate electrolytes (e.g., LiFSI-FEC/FEMC) into non-polar stable solvents. Xiulin Fan (ChBE Assistant Research Scientist), Xiao Ji (ChBE Post-doctoral Research Associate) and Long Chen (ChBE Post-doctoral Research Associate), served as first authors on the study published October 7, 2019 in Nature Energy.
The result of this chemistry is a safe (i.e., non-flammable) electrolyte boasting superior electrochemical stability that can function under a wide variety of temperatures, all while maintaining the same energy output of other LIBs.
"This 'super electrolyte' we've developed for lithium-ion batteries has an operational temperature range from -95C/-139F to 70C/158F, meaning we've created the first highly reversible battery capable of operating anywhere on Earth — from Antarctica to Death Valley, Ca," said Fan.
"The key is the all-fluorinated electrolyte, which we dissolved in a non-polar fluorine 'bath,' thereby deactivating the link between the fluorinated polar solvent and the ions," said Ji.
This process increases the energy output of the battery, while bolstering safety, voltage (5.6V) and temperature operation range. Moreover, it is cheap and simple to manufacture, making it an attractive alternative to other LIBs currently on the market.
For additional information:
Fan, X., et al. (2019). "All-temperature batteries enabled by fluorinated electrolytes with non-polar solvents," Nature Energy. DOI: 10.1038/s41560-019-0474-3.
Published October 7, 2019