News Story
New Sustainable Zinc Battery Design Could Address Future Energy Needs
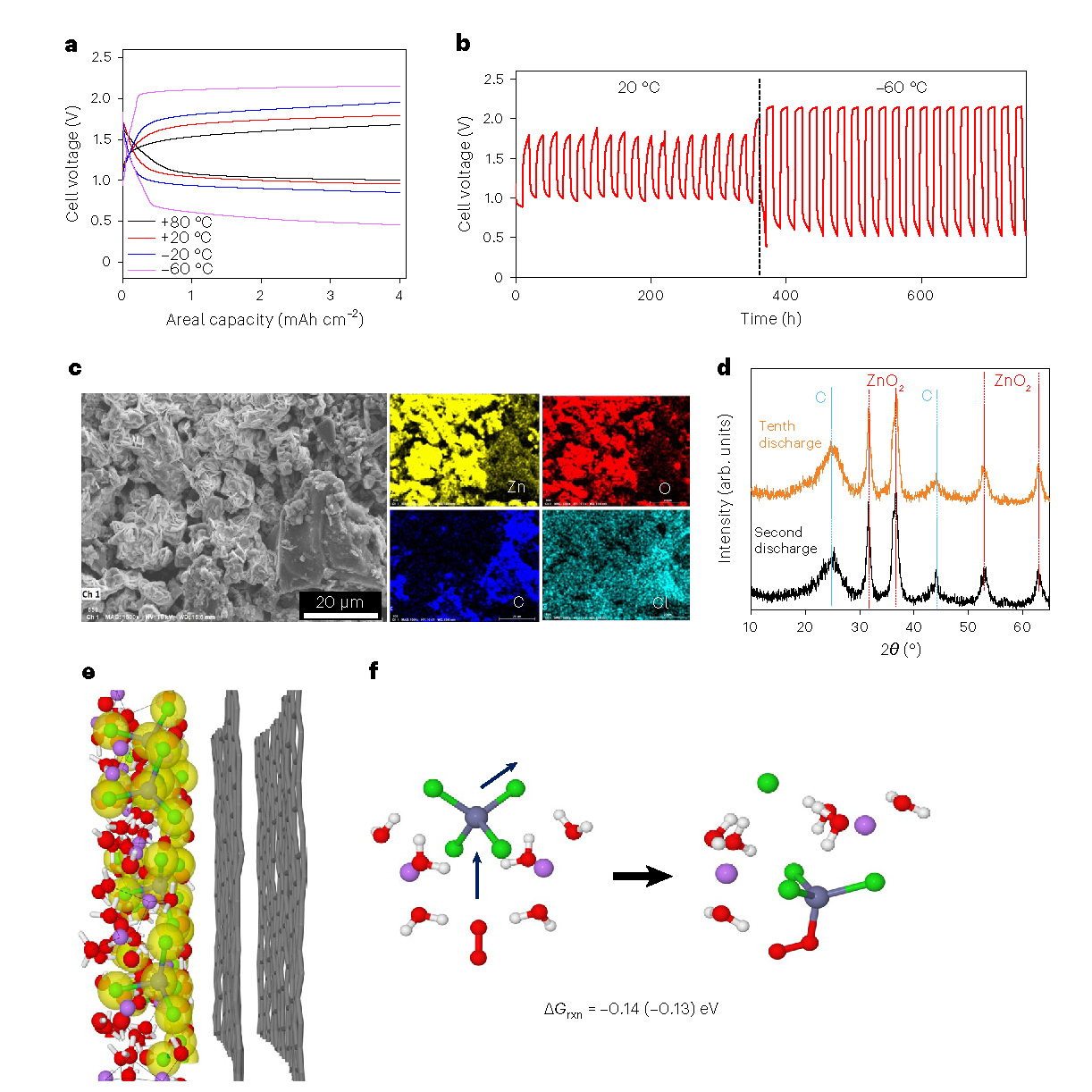
Zn–air cell performances.
With the ever-growing global need for batteries and next-generation power solutions, there is also a growing need for developing solutions that are sustainable for the growing demands. Lithium-ion battery technology has become a front-running in this space, however, they have limitations in terms of their safety as well as cost for large-scale energy storage.
To address this growing need, a research team led by Chunsheng Wang, Robert Franklin and Frances Riggs Wright Distinguished Chair Professor, Department of Chemical and Biomolecular Engineering at University of Maryland (UMD) and UMD Director of Center for Research in Extreme Batteries developed a new approach using aqueous zinc batteries. Their work was recently published in Nature Sustainability.
Aqueous zinc batteries are an emerging technology in the battery space that offers a less resource-intensive solution to the growing power demands. Zinc is relatively abundant, and because they use an aqueous-based electrolyte, they are far less combustible, and thus safer. However, their use has been limited due to their less energy-efficient nature, based on existing models, and their poor performance at low temperatures.
To address these shortcomings, the team developed a high-entropy electrolyte design that improved both the battery’s stability over existing models, as well as improving the battery’s operational performance over a wider range of temperatures.
“We introduced LiCl into the aqueous ZnCl2 to break up extended [ZnCl4]n (n>3) into shorter aggregates (n≤ 3), leading to an Li2ZnCl4·9H2O electrolyte with a unique frustrated solvation structure,” explained Chongyin Yang, former UMD Postdoctoral Research Associate and lead researcher on the paper. “Thus, we were able to achieve both a wide electrochemical stability window, better than water-in-salt electrolytes, and an exceptional operating temperature range from −80 °C to +80 °C.”
Wang adds that their idea of high-entropy may inspire the future development of electrolytes for multi-valence metal-ion batteries.
Since the electrolytes used contain chloride, corrosion issues may need to be addressed by anticorrosion coating, inhibitors, or carbonized current collectors for future applications.
This multi-institutional study included research teams from Dalhousie University, the Army Research Laboratory, the National Institute of Standards and Technology, Washington University, Brookhaven National Laboratory, Argonne National Laboratory, and the City University of Hong Kong.
For Additional Information
C. Yang, J. Xia, C. Cui, T. P. Pollard, J. Vatamanu, A. Faraone, J. A. Dura, M. Tyagi, A. Kattan, E. Thimsen, J. Xu, W. Song, E. Hu, X. Ji, S. Hou, X. Zhang, M. S. Ding, S. Hwang, D. Su, Y. Ren, X.-Q. Yang, H. Wang, O. Borodin, C. Wang, All-temperature zinc batteries with high-entropy aqueous electrolyte, Nature Sustainability, 2023. https://doi.org/10.1038/s41893-022-01028-x
Published January 23, 2023