News Story
ChBE Reasearchers Offer New Salt-Water-Based Battery Chemistry
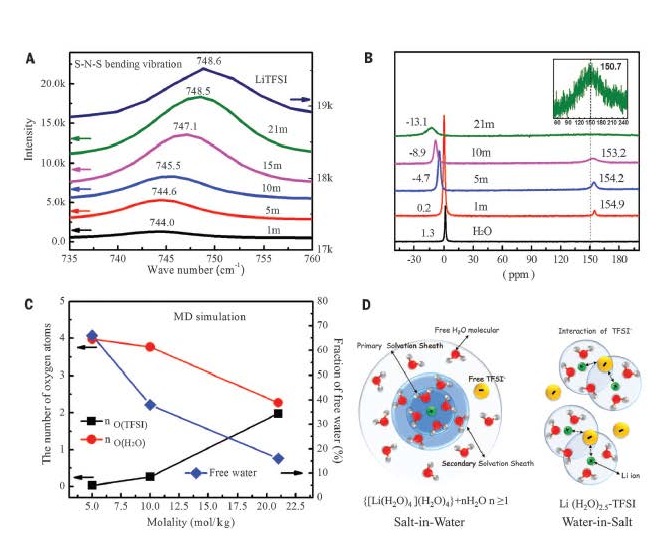
Li-ion batteries are prevalent in the consumer electronics market, however, these batteries raise concerns over their safety and environmental impact. Last year, there were several reports regarding the explosion of Samsung Galaxy smartphones in addition to the Tesla Model S incident, which killed two passengers after the vehicle burst into flames. All of these incidents were due to faulty battery chemistry – specifically, highly flammable electrolytes within Li-ion batteries.
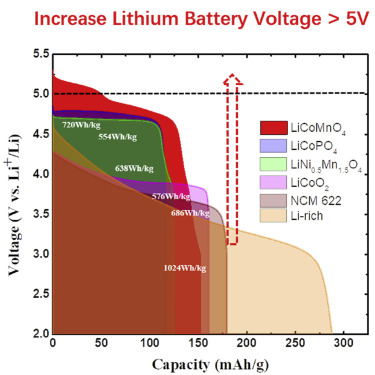
To that end, researchers in the Chemical and Biomolecular Engineering (ChBE) Department at UMD are collaborating with scientists at Army Research Lab (ARL), pushing hard to develop a safer and more efficient battery. Drs. Chongyin Yang and Chunsheng Wang at UMD, along with Drs. Kang Xu and Oleg Borodin at ARL, have produced a unique salt-water gel polymer electrolyte. When coupled with a sulfur anode and Li-ion cathode, this battery chemistry has the highest energy density ever achieved by an aqueous battery: ~ 200 Wh/kg vs. 80 Wh/kg (see Fig. 4c for chemistry comparison). The nature of this battery makes it long-lasting and completely stable.
“Fundamentally, we discovered that lithium polysulfide is thermodynamically phase-separated from the super-concentrated aqueous electrolyte,” said Chongyin Yang, a ChBE Assistant Research Scientist and first author on the study. “It is the key to enable the sulfur chemistry with high energy density and reversibility in our system.”
Indeed, this battery chemistry could offer a wide range of practical applications in our current digital lifestyle.
“Prior to this work, no one thought it possible to fully utilize a solid sulfur electrode in water-based batteries, since polysulfides are highly soluble in water and tend to form hydrogen sulfide,” said Dr. Wang. “Thanks to the discovery of thermodynamic phase-separation and the suppressed activity of water, our Li-ion/sulfur battery proceeded with fast kinetics that significantly differ from those in non-aqueous systems – polysulfides insolubility in an aqueous electrolyte essentially eliminates the parasitic shuttling.”
The next step for the group is to identify an alternate cathode chemistry with a higher energy density, which would increase the overall energy density of full cells, eventually challenging current commercial non-aqueous, Li-ion batteries.
This research, entitled, “Unique aqueous Li-ion/sulfur chemistry with high energy density and reversibility,” was published in the Proceedings of the National Academy of Sciences (PNAS) in June, 2017.
Published June 30, 2017