Press Release
Nanoscale Imaging Method Offers Insight into Alloyed Nanoparticle Synthesis
UMD research group visualizes how organic ligands contribute to bimetallic nanoparticle synthesis.
FOR IMMEDIATE RELEASE February 10, 2021
CONTACT:
Katie Doyle
301 405 0379
khollan3@umd.edu
Alloyed nanocrystals. Credit: Mei Wang for UMD.
Catalysts, often metal nanoparticles, are involved in the production of over 80% of commercial products such as plastics, fuels and pharmaceuticals. Computational methods aid in designing nanoparticle catalysts consisting of mixtures of metals, called alloyed nanoparticles, with high reaction activity and selectivity. However, producing alloyed nanoparticles with arbitrary composition in the lab do not yet exist. Indeed, the fundamental chemistry of alloyed nanoparticle synthesis remains an enigma.
To that end, a research team at the University of Maryland (UMD) led by Taylor Woehl, an assistant professor in the Department of Chemical and Biomolecular Engineering (ChBE), applied a novel method – in situ liquid phase transmission electron microscopy (LP-TEM) synthesis – allowing a closer look at the molecular and nanoscale processes that govern how metals mix into alloyed nanoparticle during wet chemical synthesis. Mei Wang, a ChBE Ph.D. Student, served as first author on the study, published in ACS Nano.
"We observed the formation of nanoparticles made of gold and copper – promising catalysts for converting CO2 into valuable organic molecules – in real time at the nanometer length scale," Wang said. "With this method, the synthesis of nanoparticles is achieved by irradiating a liquid precursor with high energy electrons to simulate the conditions of wet chemistry. We found electron synthesis conditions that closely mimicked wet chemical synthesis, which was surprising given that the radiation dose the sample receives is many times greater than in a commercial nuclear reactor."
By discovering these conditions, the authors ensured that what they saw with LP-TEM was representative of what occurs during wet chemical synthesis on the benchtop. Reaction simulations showed that organic ligands in the solution, normally used to control the size and stability of the nanoparticles, protect the reaction solution from being damaged by the high energy electrons.
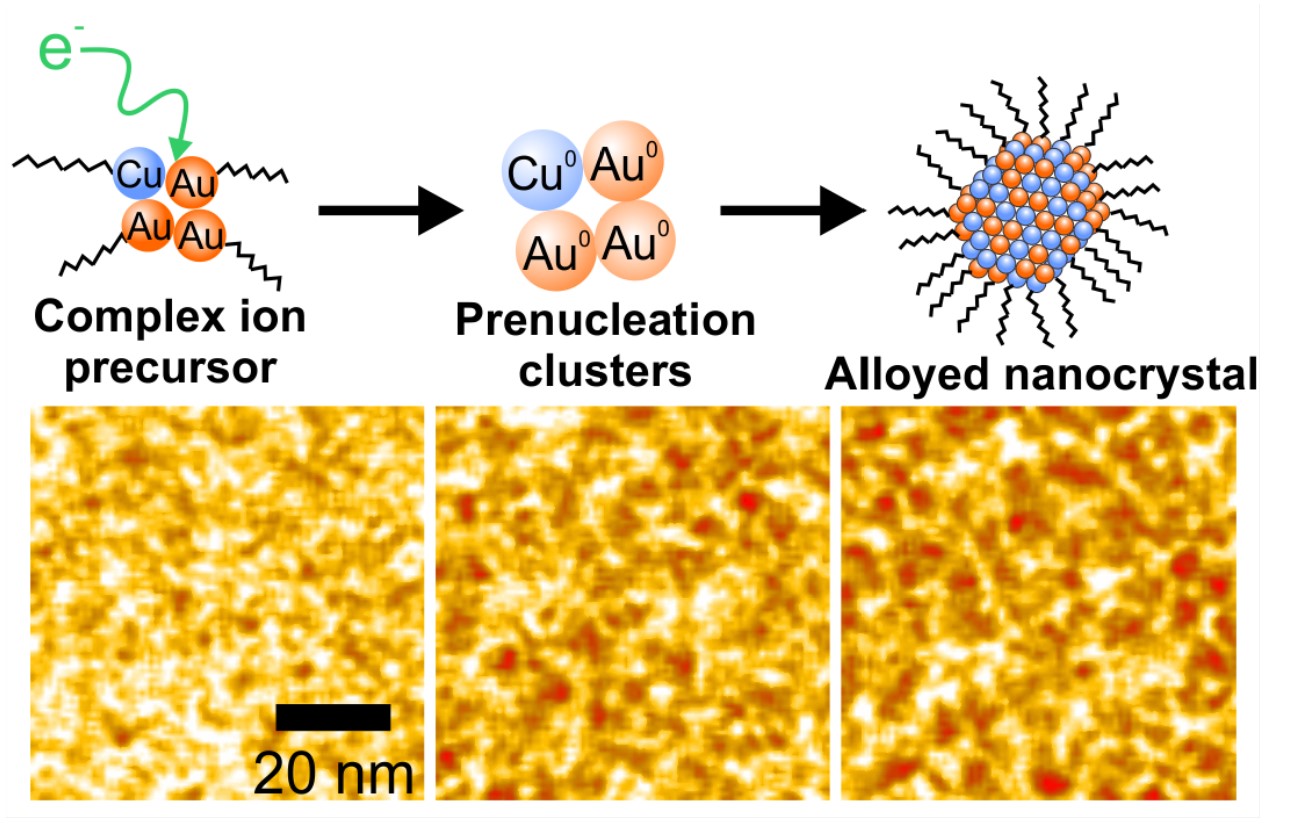
A key observation in the study was that the presence of an organic ligand was critical to combining gold and copper into well mixed alloyed nanoparticles.
"We found that the ligand enabled alloy formation by covalently bonding to gold and copper to form complex ions," said Woehl. Atomic resolution imaging and mass spectrometry showed that the complex ions were converted to intermediate species in the synthesis reaction, called prenucleation clusters. We found these clusters, each consisting of a few gold and copper atoms, were critical to forming an alloy."
The intermediate species then assembled together into nanocrystals with a similar composition. This nanocrystal formation pathway is distinct from the classical picture of single atoms congregating into a nanoparticle.
The authors found that the organic ligands play an important secondary role of encouraging the formation of prenucleation clusters containing both gold and copper atoms. These findings suggest that control over metal cluster intermediates is the key to synthesis of alloyed nanoparticle catalysts.
For more information:
Wang, M., Leff, A.C., Li, Y., Woehl, T.J. (Jan 2021). Visualizing Ligand-Mediated Bimetallic Nanocrystal Formation Pathways with in Situ Liquid-Phase Transmission Electron Microscopy Synthesis. ACS Nano. DOI: 10.1021/acsnano.0c07131
About the A. James Clark School of Engineering
The University of Maryland’s A. James Clark School of Engineering is a premier program, ranked among the top 20 in the world. Located just a few miles from Washington, D.C., the Clark School is at the center of a constellation of high-tech companies and federal laboratories, offering students and faculty access to unique professional opportunities.
Our broad spectrum of academic programs, including the world’s only accredited undergraduate fire protection engineering program, is complemented by a vibrant entrepreneurial ecosystem, early hands-on educational experiences, and participation in national and international competitions.
The Clark School is leading research advancements in aerospace, bioengineering, robotics, nanotechnology, disaster resilience, energy and sustainability, and cybersecurity. From the universal product code to satellite radio, SMS text messaging to the implantable insulin pump, our students, faculty, and alumni are engineering life-changing innovations for millions. Learn more at www.eng.umd.edu.