News Story
Woehl Group Unravels Novel 'Nanochemistry' with In situ Electron Microscopy

Mei Wang adjusts a microsope in the Woehl Group lab.
Nanoparticles (NPs) - small particles with sizes ranging from 1 – 100 nm - possess fascinating optical, catalytic, electronic, and magnetic properties that emerge due to their small size and large fraction of surface atoms. Unlocking the desirable properties of NPs requires synthesizing them with uniform size, shape and surface structure; however, synthesizing them in a systematic and predictive way is quite difficult, which is why “nanochemistry” is poorly understood. Currently, chemists rely on trial and error methods to screen chemical reactions for making NPs with little fundamental understanding to guide their experiments – this makes synthesis extremely labor intensive; thus, industrial-scale production of NPs is currently not economically feasible. A better understanding of the nanochemistry of nanoparticle synthesis will enable precision synthesis of NPs without extensive trial and error experiments.
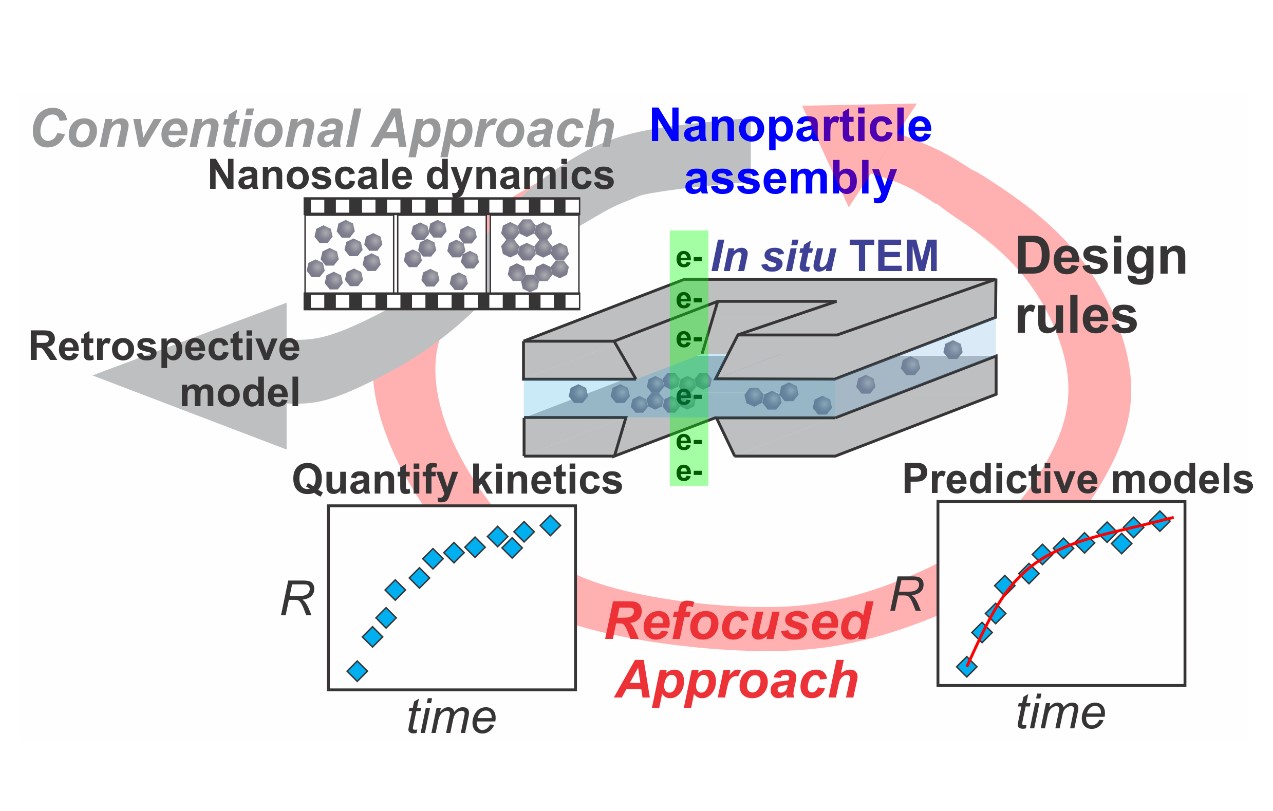
To that end, Taylor Woehl – an assistant professor in the UMD Department of Chemical and Biomolecular Engineering (ChBE) – and his research team have utilized a novel microscopy technique - liquid cell electron microscopy - to “see” silver nanoparticles as they form and obtain quantitative information about the processes these materials undergo during synthesis. ChBE Ph.D. student Mei Wang served as first author on the paper, recently published in Chemistry of Materials. Florida State University Professor, Chiwoo Park, who is an applied statistician, provided input on data mining and artificial intelligence.
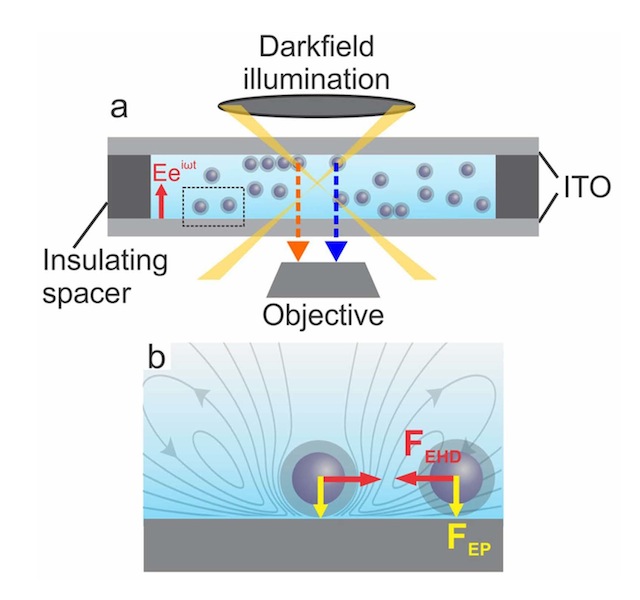
“Essentially, we create a nanoreactor inside a transmission electron microscope [TEM] that enables us to watch nanoparticles as they form from a chemical precursor solution,” said Woehl. “The experiments are performed by passing high-energy electrons through a liquid precursor solution to acquire nanometer scale resolution images of single nanoparticles as they form.
Woehl continued, “The electrons also stimulate the formation of nanoparticles by converting chemical precursors - in this case silver ions - into nanoparticles. The chemistry of this process - which we term ‘electron beam nanochemistry’ - is still poorly understood.”
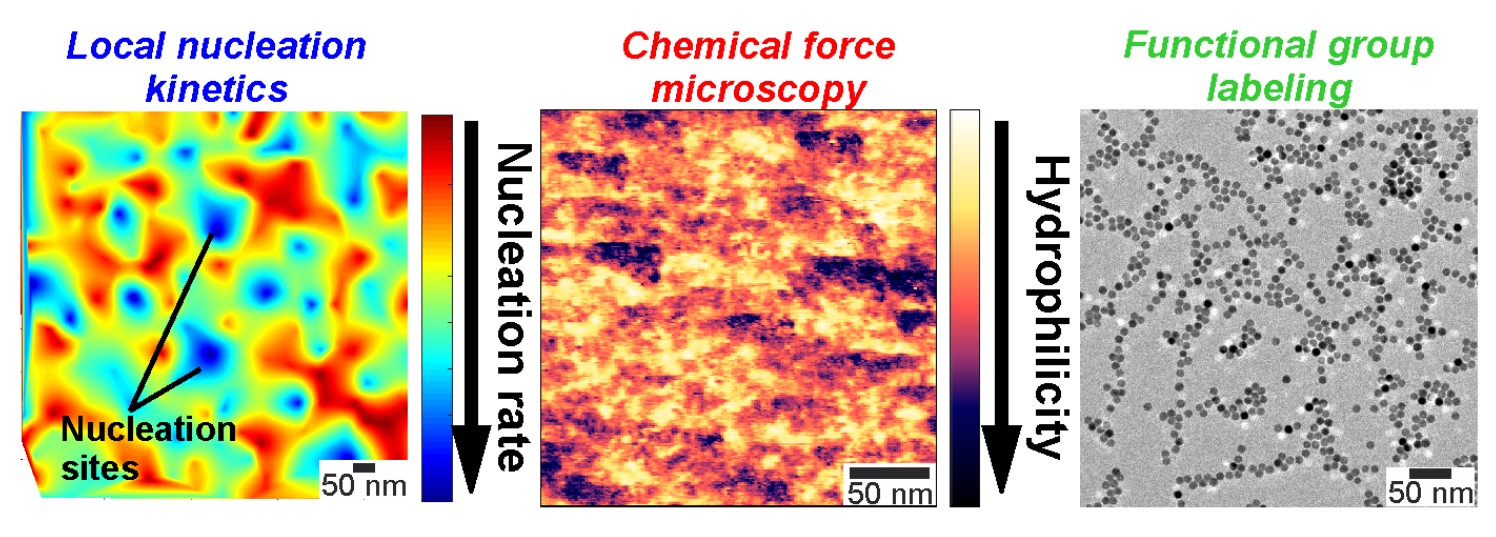
Through a combination of data mining, numerical kinetic simulations and reaction kinetics, the group showed how the chemistry of the precursor solution impacts the formation kinetics of the silver nanoparticles. The results were counter-intuitive.
“We found that increasing the intensity of the electrons passing through the sample actually led to slower formation kinetics as opposed to faster kinetics as previously thought,” said Woehl. “These results counter all prior results, and will allow us and other microscopists, to design experiments that systematically probe the formation kinetics of nanoparticles.”
The group also developed a reaction-kinetic model, enabling them to quantify the dynamics of NP formation at the near-atomic level.
“The reaction kinetic model enables us to unravel the nucleation and growth kinetics, and probes nucleation kinetics occurring at the sub-nanometer scale, which are normally not accessible with Liquid Cell Electron Microscopy (LCEM),” said Mei Wang. “The model should also be sufficiently general to capture the formation kinetics of other metal nanocrystals, since it accounts for specific aspects of LCEM experiments.”
In the future, they will be able to use nanochemistry to design experiments to probe and quantify specific material processes involved in NP formation, such as precursor reactions, ligand binding, nucleation and growth.
“Previously, these processes have been described based on qualitative models, but our alternative approach brings together powerful quantitative chemical engineering concepts, shedding new light on nanoparticle synthesis,” said Woehl. “Knowledge gained by our experiments can be translated from the electron microscope to the chemist’s beaker, enabling improved synthesis of next generation nanoparticles.”
For additional information:
Wang, M., Park, C., and Woehl, T.J. (2018). Quantifying the Nucleation and Growth Kinetics of Electron Beam Nanochemistry with Liquid Cell Scanning Transmission Electron Microscopy. Chemistry of Materials, 30 (21), 7727-7736. DOI: 10.1021/acs.chemmater.8b03050.
Published November 30, 2018