News Story
Less Precious: Big Copper Particles Could Make Your Electronics Cheaper
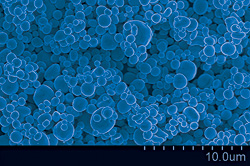
Oxide-free, micrometer sized Copper particles generated by a spray pyrolysis process with a cosolvent of ethylene glycol at 1000°C. These hollow, smooth-surfaced particles were designed to replace the precious metal particles used in conductive pastes, which are essential to the electronics industry.
Base metals, such as copper and nickel, are good conductors of electricity and heat and cost far less than gold, but unlike precious metals are subject to oxidation, which results in a drop in conductivity. If pure, non-oxidized base metal powders could be produced for use in conductive pastes, the cost of electronics and solar cells could be reduced. ChBE professor and chair Sheryl Ehrman and her research group have not only discovered a way to manufacture oxide-free copper particles with the required properties, but also a way to do it relatively safely, and without toxic byproducts.
There are two common approaches to creating oxide-free powders, Ehrman explains, and neither is ideal. "You can dissolve your precursor in a solvent and conduct a precipitation reaction that results in the powder settling out, but that leaves you with a vat of contaminated solvent," she explains. "Or, you can make a mist out of an oxidized metal precursor and reduce it to a pure metallic powder in a furnace by adding hydrogen gas, but the reaction requires adding hydrogen at levels that create an explosive mixture.
Ehrman's group developed a new technique in which an organic compound, a double alcohol called ethylene glycol, is added to an aqueous solution of copper salts. The solution is converted to a mist inside a furnace. As the ethylene glycol decomposes in the heat, it produces a small amount of hydrogen—enough to reduce the copper salts to pure copper particles, at a temperature below hydrogen's flammability limit.
"The reaction doesn’t seem to create any measurable amount of carbon monoxide, which is good," says Ehrman. "It creates hydrogen in situ but the levels remain below the lower flammability limit. The final product is just a dry powder, so there’s no contaminated solvent stream to get rid of, and it's a safer process because the reaction environment isn't explosive."
The copper particles produced by the reaction are both smooth and "big," measured at he micro- rather than nanoscale. Ehrman stresses that while the trend in electronics increasingly leans toward intricate products created at the nanoscale, in this situation, bigger and smoother are better.
"DuPont will mix the particles in with a other materials to make the conductive paste," she explains. "In the end it’s supposed to melt and flow when fired, so we don’t want our particles to have the porosity or high surface area nanoscale structures have, because we don't want nooks and crannies the other ingredients can get into. We want the dominant ingredient in the paste to be metal, with as little binder or filler as possible."
The oxide free copper particle research was funded by DuPont and by the National Science Foundation’s Grant Opportunities for Academic Liaison with Industry program. Ehrman's co-authors on the work were George Peabody (B.S. '12, chemical engineering), ChBE graduate student Kai Zong, and Howard Glicksman (Department of Microcircuit Materials, DuPont Electronic Technologies).
For More Information:
See: Kai Zhong, George Peabody, Howard Glicksman and Sheryl Ehrman. "Particle generation by cosolvent spray pyrolysis: Effects of ethanol and ethylene glycol." Journal of Materials Research, Available on CJO August 2012. dx.doi.org/10.1557/jmr.2012.248.
Visit Professor Ehrman's web site »
Published August 23, 2012









