News Story
Hybrid Electrolyte Bridges Gap Between Aqueous and Non-aqueous Battery Technology
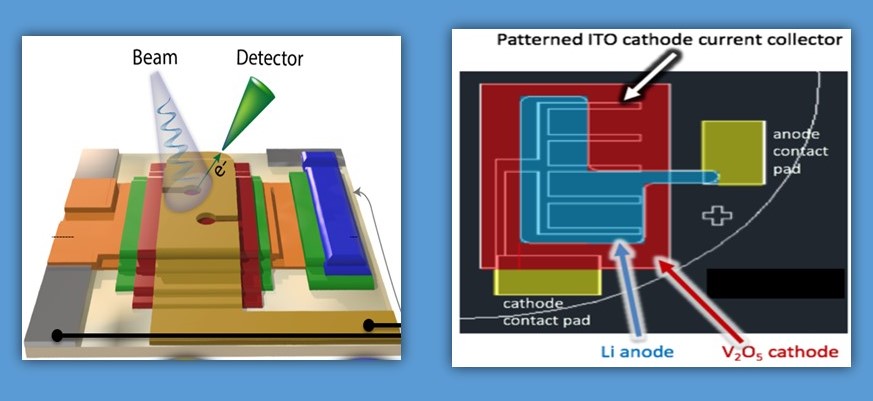
Hybrid aqueous/non-aqueous electrolyte (HANE) inherits the merits from both aqueous (non-flammability) and non-aqueous (high electrochemical stability) systems. Performance comparable with state-of-the-art- Li-ion batteries.
The demand for portable power sources has increased exponentially over the last two decades. Rechargeable batteries – most especially, lithium batteries (LiBs) – are so fundamental to modern society that it’s hard to imagine life without them. Sustainability concerns, too, have increased in line with the demand for portable power. Indeed, there are numerous research teams developing alternatives to LiBs – lithium-air, lithium-metal, ceramic, and various water-based (i.e., aqueous) designs to name a few – that boast higher energy densities, lower production cost, quick re-charge rates, and that are environmentally friendly.
A collaborative research team between the University of Maryland (UMD) Department of Chemical and Biomolecular Engineering (ChBE) and U.S. Army Research Lab (ARL), led by Professor Chunsheng Wang (UMD) and Dr. Kang Xu (ARL), has developed a novel aqueous/non-aqueous chemistry – which blends the strongest solvents from both water-based and non-water-based designs – to create a hybrid electrolyte that is highly efficient, cost-effective, eco-friendly and boasts a high energy density. This hybrid aqueous/non-aqueous electrolyte, or HANE, is also non-toxic and non-flammable, which will relax the safety concerns currently associated with the conventional Li-ion battery (i.e., Tesla and Samsung debacles).
This study, entitled, “Hybrid Aqueous/Non-aqueous Electrolyte for Safe and High-Energy Li-ion Batteries,” was published online March 12 in Joule. Dr. Fei Wang, an UMD/ARL joint post-doctoral researcher, served as the paper’s first author.
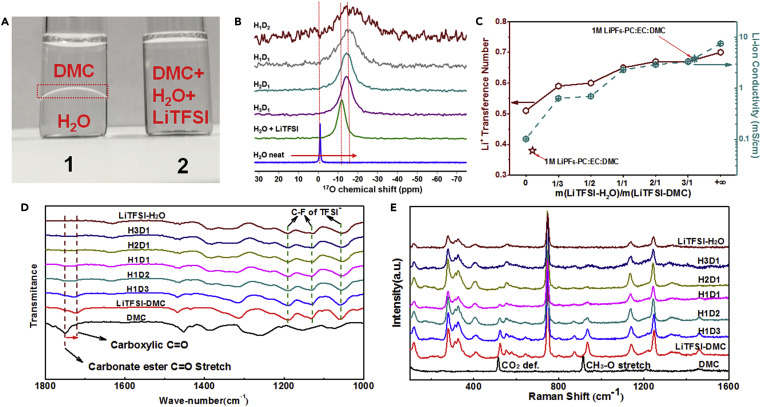
In conducting this research, “we were motivated by the fact that the solid electrolyte interphase derived from the decomposition of the TFSI anion could extend the stability window of the water, which was reported by our group in Science,” Fei Wang stated. “To further extend the window, we introduced an organic component that not only rearranges the interfacial structure near the inner-Helmholtz layer, but that could also decompose to form an additional SEI component. Based on the composite interphases, the narrow thermodynamic stability window of the water (1.23 volts) was expanded to a 4.1 volts, which could support more electrode material choices leading to a higher energy density.”
A unique characteristic of this design is that it can be manufactured in open-air.
“Conventional Li-ion batteries, by comparison, require a rigorous moisture-free environment – a dry-room is a must,” Professor Wang explained. “The resultant batteries have to be hermetically-sealed making them rather rigid, which essentially eliminates any flexibility.” The beauty of HANE is that it renders batteries unprecedented freedom from such rigidity. “We imagine that in the future, batteries can be designed to work in open atmosphere, or printed into any shape required by individual devices,” said Professor Wang.
This new technology, having numerous applications, still has one main obstacle: “the electrolyte needs several (charge/discharge) cycles to form a stable SEI on the anode surface, which sacrifices some energy density,” Fei Wang explained.
“In the future, we will do some pretreatment on the anode surface to shorten or remove the formation process," Dr. Xu added. "It’s still a work in progress, but we hope to commercialize this product in the near future.”
For additional information:
Wang et al. Hybrid Aqueous/Non-aqueous Electrolyte for Safe and High-Energy Li-ion Batteries, Joule (March, 2018), https://doi.org/10.1016/j.joule.2018.02.011
Published March 12, 2018