News Story
Hong, Collaborators Create Liposome-Hydrogel Hybrids for Drug Delivery
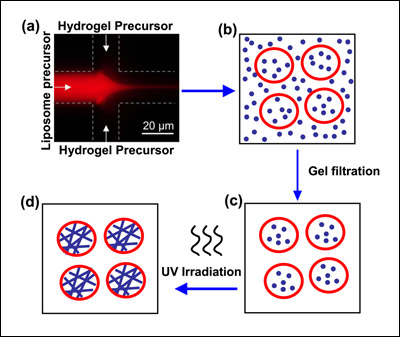
Schematic depicting the creation of liposome-hydrogel hybrids. A solution containing phospholipid ("liposome precursor") mixes with a solution containing hydrogel precursor (a). Blending together at the interface of the two channels, the phospholipid forms liposomes (b) that trap the hydrogel precursor inside. Material outside the vesicles is removed (c) and the liposomes are UV irradiated. This polymerizes the protein chains in the hydrogel and yields a liposome-hydrogel hybrid (d). Image credit: NIST.
The project was led by Fischell Department of Bioengineering graduate student (now alumna) Dr. Jennifer Hong (Ph.D. '10), advised by Department of Chemical and Biomolecular Engineering associate professor Srinivasa Raghavan. Her collaborators included Raghavan, Michael Gaitan (NIST), Silvia H. DePaoli Lacerda (FDA), Laurie E. Locascio (NIST), and Samuel M. Stavis (NIST)
In a paper recently published in the journal Langmuir, the researchers reviewed how liposomes and hydrogel nanoparticles have individual advantages and disadvantages for drug delivery. While liposomes have useful surface properties that allow them to target specific cells and pass through membranes, they can rupture if the surrounding environment changes. Hydrogel nanoparticles are more stable and possess controlled release capabilities to tune the dosage of a drug over time, but are prone to degradation and clumping. The group's goal was to engineer nanoparticles incorporating both components to utilize the strengths of each material while compensating for their weaknesses.
To manufacture their liposome-hydrogel hybrid vesicles, the researchers adapted a NIST-University of Maryland technique known as COMMAND (COntrolled Microfluidic Mixing And Nanoparticle Determination) that uses a microfluidic device. In the new work, phospholipid molecules are dissolved in isopropyl alcohol and fed via a tiny inlet channel—only 21 micrometers in diameter, or three times the size of a yeast cell—into a "mixer" channel, then "focused" into a fluid jet by a water-based solution added through two side channels. Hydrogel precursor molecules are mixed in with the focusing fluid.
As the components blend together at the interfaces of the fluid streams, the phospholipid molecules self-assemble into nanoscale vesicles (spheres with hollow centers) of controlled size and trap the monomers in solution inside. The newly formed vesicles are then irradiated with ultraviolet light to polymerize the hydrogel precursors they carry into a solid gel made up of cross-linked chains. These chains give strength to the vesicles while permitting them to retain the spherical shape of the liposome envelope (which, in turn, would facilitate passage through a cell membrane).
To turn the liposome-hydrogel hybrid vesicles into cellular delivery vehicles, a drug or other cargo would be added to the focusing fluid during production.
Story adapted from the original NIST press release, courtesy of Michael E. Newman.
For More Information:
"NIST, Maryland Researchers COMMAND a Better Class of Liposomes" »
Visit Professor Raghavan's Complex Fluids and Nanomaterials Group web site »
Published July 22, 2010