News Story
UMD Researchers Offer Solution to Volatile Battery Chemistry in Electronics

Salt-water-based gel electrolyte atop a Li metal.
The safety of batteries used in electronics and electric vehicles is still a critical concern for consumers and manufacturers alike. Aqueous batteries could have provided safe solutions to typical Lithium-ion batteries, but lack the ability to support the aggressive chemistries of Li-ion batteries, until now.
Researchers at the University of Maryland (UMD), partnered with the U.S. Army Research Laboratory (ARL), have developed a 4.0-volt aqueous Li-ion battery based on water-in-salt concept that is capable of powering household electronics, including mobile phones and laptop computers. This research will be published in the first edition of the journal Joule on September 6, 2017.
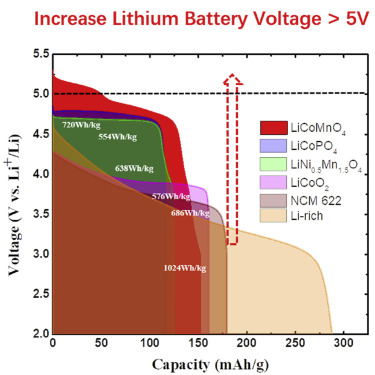
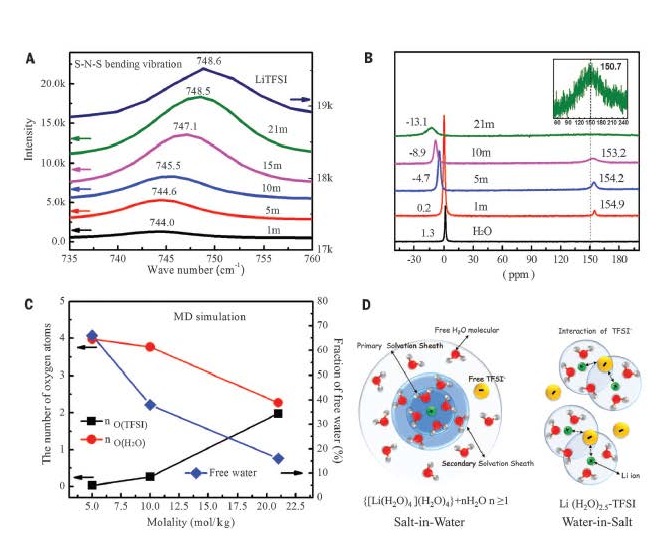
“Aqueous batteries are indeed safe, but the energy density is low due to a voltage of less than 2.0V for the water-based electrolytes,” said Chunsheng Wang, professor of Chemical and Biomolecular Engineering (ChBE) at UMD. “We designed a hydrophobic organic gel electrolyte to the surface of the anode, which and repels water away from the electrode surface and then transformed into a dense solid electrolyte interphase in the first charge. By doing so, the hydrogen evolution due to water reduction [i.e., the cathodic challenge] is prohibited.”
“High energy density and safety are two conflicting issues of current Li-ion batteries. Aqueous electrolyte can best compromise these two by removing the flammable electrolyte out of the equation,” said Kang Xu, lab fellow and team leader at ARL. “In this work, aqueous Li-ion battery can achieve 4 V – the benchmark of the voltage for high-energy Li-ion battery, which make us really excited about the promising large-scale applications of aqueous Li-ion batteries.”
Prior to this work, Dr. Wang, Chongyin Yang at CHBE UMD, and Drs Kang Xu, Oleg Borodin at ARL, published the precursor to this research at Science and Proceedings of National Academy of Sciences, in which they discussed the development of a 3.0V battery and how they might increase the energy density of water-based battery chemistry.
“We are so glad that we are moving forward – further pushing the voltage and energy density limits to the level of state-of-art non-aqueous Lithium-ion batteries,” said Dr. Chongyin Yang, a ChBE assistant research scientist and first author of this work. “Our design of unique protection layer on anodes was proved as a successful strategy to utilize graphite or lithium metal in our previous aqueous electrolyte system. More effects are needed to optimize it, but future is bright.”
For additional Information:
Joule, Yang et al.: "4.0 V Aqueous Li-ion Batteries" http://www.cell.com/joule/fulltext/S2542-4351(17)30034-X , DOI: 10.1016/j.joule.2017.08.009
Published September 7, 2017